BACK
 Glide at Static Pressure
Glide at Static Pressure
 Does Refrigerant Get Old or Wear Out?
Does Refrigerant Get Old or Wear Out?
 What Is Temperature Glide?
What Is Temperature Glide?
#glide
Tech Tips:
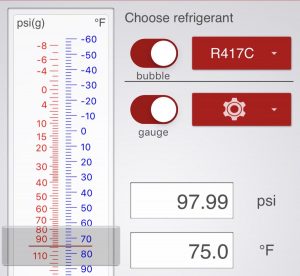
Source: Refrigerant Slider in the Ref Tools App We've been pretty spoiled in residential and light commercial HVAC in the USA because we haven't needed to deal with glide much. In HVAC/R, glide is the window of difference between the boiling points in blended refrigerants; when you have a refrigerant blend made up of different […]
Read more
Over the years, I have heard technicians say that refrigerant can wear out or “lose its blend” by sitting in a tank. Refrigerant does not wear out or “lose its blend”… at least not like that. What can and does happen is called “fractionation.” Refrigerant blends are composed of a mix of refrigerants with different […]
Read more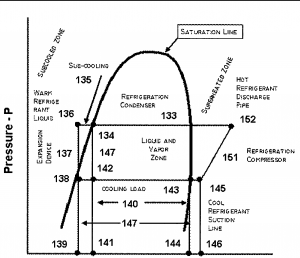
We've all heard about glide, but what is it really, and how does it affect our system? Glide, or temperature glide, is the difference between the bubble point and the dew point of the zeotropic refrigerant mixture. Well, that wasn't very helpful, was it? All we did was introduce new terms without defining them, […]
Read more






