BACK
 Does Air or Nitrogen Absorb Water?
Does Air or Nitrogen Absorb Water?
 Brazing in TXVs: Best Practices
Brazing in TXVs: Best Practices
 Should I Pump Down or Recover?
Should I Pump Down or Recover?
 Two Tips for Tough Vacuum Jobs
Two Tips for Tough Vacuum Jobs
 Start Flowing Nitrogen Sooner
Start Flowing Nitrogen Sooner
 Non-non-condensables
Non-non-condensables
 Sweeping with Nitrogen
Sweeping with Nitrogen
 How (and Why) to Flow Nitrogen While Brazing
How (and Why) to Flow Nitrogen While Brazing
 Triple Evacuation and Nitrogen FACTS
Triple Evacuation and Nitrogen FACTS
 Q&A – Vacuum Questions Answered
Q&A – Vacuum Questions Answered
 Things to Keep out of the System – Class
Things to Keep out of the System – Class
 Flow Nitrogen Great Again
Flow Nitrogen Great Again
#nitrogen
Tech Tips:

There are many examples of teaching using metaphors to help someone grasp how something works without being EXACTLY correct. Some examples are how we often use water flow to explain electrical flow or refrigerant circuit dynamics. It's enough like the way it works to get our heads wrapped around it, but there are many differences. […]
Read more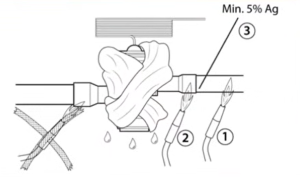
This is a quick tip from the “Expansion Valves – What Does and Doesn't Matter?” livestream on our YouTube channel featuring Joe Shearer, Matthew Taylor, and Corey Cruz. It’s time to talk about brazing again! Even though rehashing “flow nitrogen” and “use a wet rag” gets a little old, bad TXVs and callbacks also get […]
Read more
During my second year in HVAC, the company I worked for was hired to fix some poor work that was done on a multi-head inverter system. The system used flare connections, and many of them were leaking. The system also had a branch box, which meant there were even MORE flares. So we pumped all […]
Read more
In the last year or so, I have had a couple of puzzling experiences related to pulling a vacuum. These instances have left me with questions and clues where I thought I had given up on understanding a certain vacuum situation. Typically, if I’ve had a hard time achieving a proper vacuum, I would be […]
Read more
My technician (and brother-in-law), Bert, made a good point. (It's hard for me to admit it, but it's true.) When he needs to open the refrigerant circuit to make a repair, regardless of whether he is recovering or pumping down, he pulls out his nitrogen tank and his regulator. (We like the Western Enterprises VN-500 […]
Read more
Flowing nitrogen while brazing and pressurizing with nitrogen are great practices. Putting nitrogen in with the refrigerant? Not so much. Nitrogen is a “non-condensable” gas because it cannot be condensed (under normal conditions). However, nitrogen is just one of the non-condensables. First, let's talk about what a non-condensable gas is. Any gas that does […]
Read more
Two days prior to this article being published, I sent one out about the popular fallacy that nitrogen “absorbs” moisture. That tech tip went out at 7 PM eastern time like usual, and I was sitting on the couch watching something on the Food Network (as usual). At 7:10 PM, I get a call on […]
Read more
When I started in the trade, the idea of flowing nitrogen while brazing was nothing more than the punchline of a joke. Like pulling a vacuum with a micron gauge or proper recovery, it was a wink and nod proposition rather than a real practice. I've had to unlearn many bad habits since those early […]
Read more
This article was written by longtime contributor and RSES CM Jeremy Smith. Thanks, Jeremy! Nitrogen doesn’t absorb moisture like many techs think that it does, and I think that we, as technicians, need to reevaluate the reasons for the “triple evacuation” process. OK. Hold on, now. Put down the pitchforks and torches, and give me […]
Read moreVideos:
Podcasts:

In this episode of the HVAC School podcast, host Bryan dives deep into the topic of vacuum in HVAC and refrigeration systems. He addresses two questions from social media about vacuum, expanding them into a comprehensive discussion of best practices, common issues, and techniques for effective vacuum procedures. Bryan starts by explaining where to […]
Read more
This podcast is a class taught by Bryan: Things to Keep Out of the System. He covers some installation best practices along the way to keep contaminants and non-condensable gases out of the system. We want to keep air, water, dirt, copper shavings, solvents, and nitrogen out of an operating system. All we want […]
Read more
In this episode of the HVAC School Podcast, Bryan talks with Tim Bagnall about flowing nitrogen. Many techs don't flow nitrogen. Some may say that it is overkill, but it has been shown that flowing nitrogen displaces oxygen while brazing and prevents harmful scale from forming on the copper. Scale is very problematic, and it will likely […]
Read more








