BACK
 Glide at Static Pressure
Glide at Static Pressure
 Fancy Refrigerant Words
Fancy Refrigerant Words
 Is It OK To Top Off With R410a?
Is It OK To Top Off With R410a?
#blend
Tech Tips:
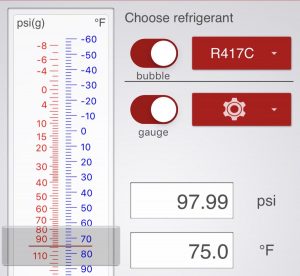
Source: Refrigerant Slider in the Ref Tools App We've been pretty spoiled in residential and light commercial HVAC in the USA because we haven't needed to deal with glide much. In HVAC/R, glide is the window of difference between the boiling points in blended refrigerants; when you have a refrigerant blend made up of different […]
Read more
The HVAC industry uses all sorts of fancy words to classify refrigerant. As such, there are all sorts of complicated refrigerant acronyms: HFC, HCFC, CFC. Let's also not forget the mythical zeotropic, azeotropic, and near-azeotropic descriptors. Let's simplify those. (Though if you want to go back to the basics first, check out this article on […]
Read more
I am consistently surprised by how much false information still circulates out in the field, and one of the ones I hear often is the idea that you cannot or should not “top-off” or recharge R410a systems on top of an existing charge of R410a when the system is low. So, to be clear, before […]
Read more





