BACK
 Adiabatic Cooling, Blower Settings, and Why You Care
Adiabatic Cooling, Blower Settings, and Why You Care
 The Impact of Adding or Removing Water From Air
The Impact of Adding or Removing Water From Air
 A Bit of Adiabatic Air Science For Techs – FB Live Video
A Bit of Adiabatic Air Science For Techs – FB Live Video
#adiabatic
Tech Tips:

Just so you don't get bored and quit reading, let's get straight to the point. When the blower runs for more than a few minutes after the system has cycled off in cool mode, the air may continue to be “cooler” (lower sensible temperature) coming out of the supply. However, the heat content of the […]
Read more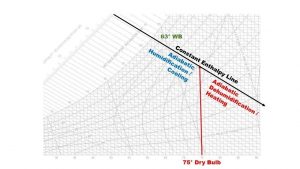
Air conditioning was about humidity control from the very start. Willis Carrier's very first air conditioning system was all about controlling the humidity with the side effect that it also could reduce the sensible temperature. Theaters caught on that this newfangled contraption could lead to big summer numbers when they installed it to keep patrons […]
Read more
In this video Bryan talks about Heat (Enthalpy) Adiabatic cooling Latent and Sensible heat Evaporative Cooling Humidity Blower Off Delay
Read more






